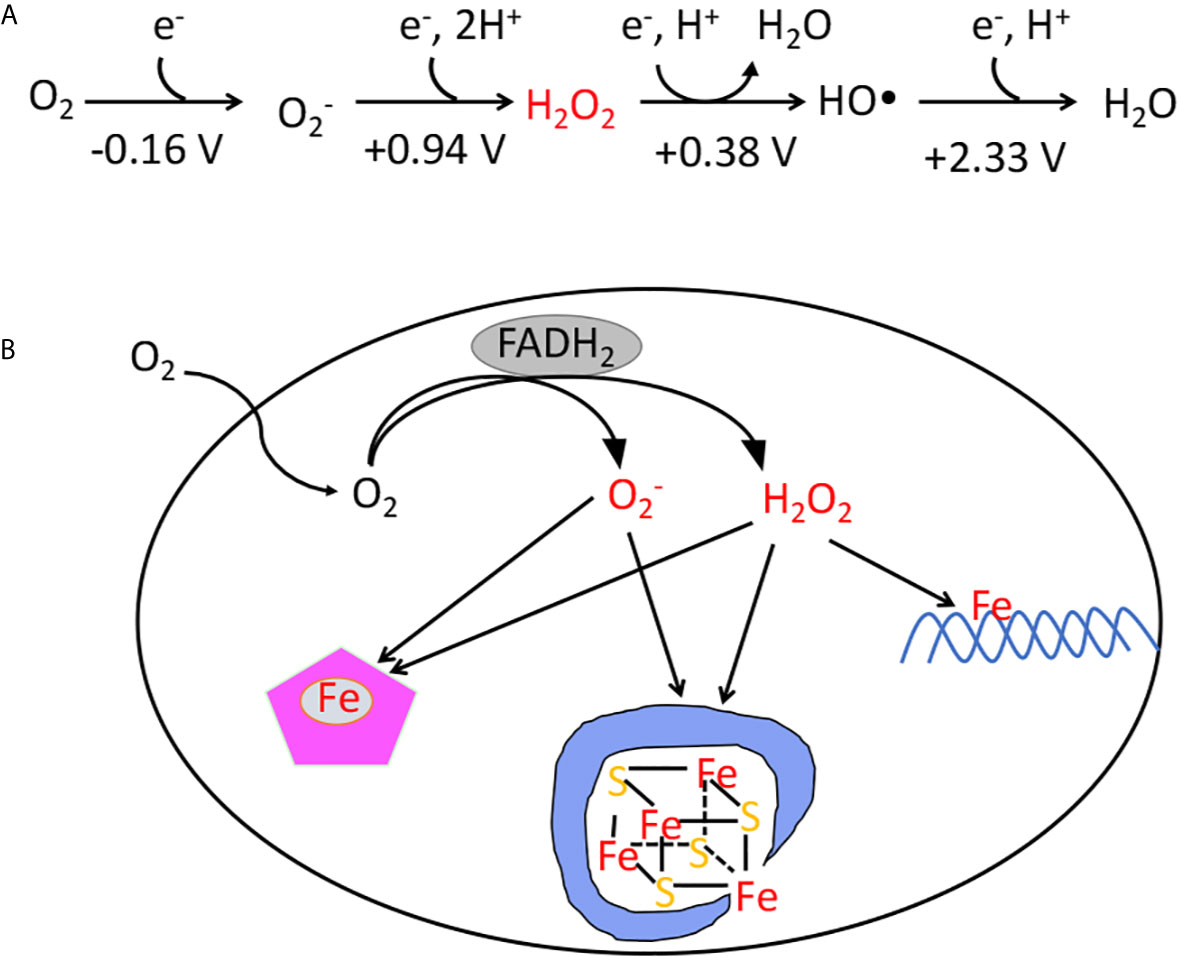
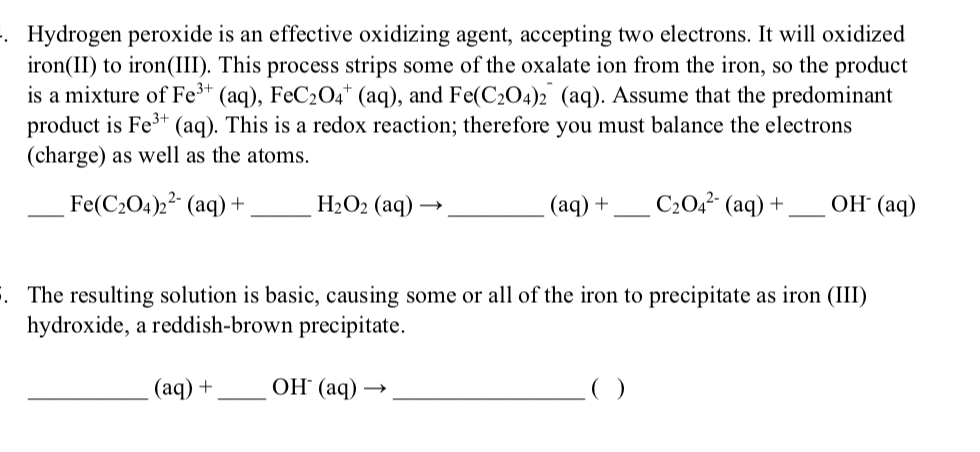
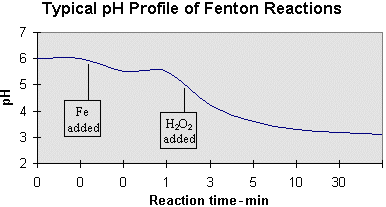
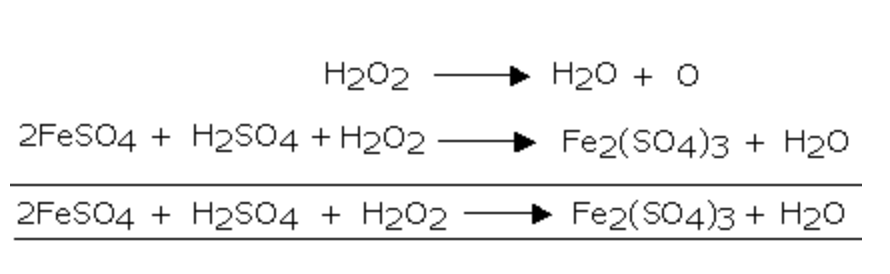
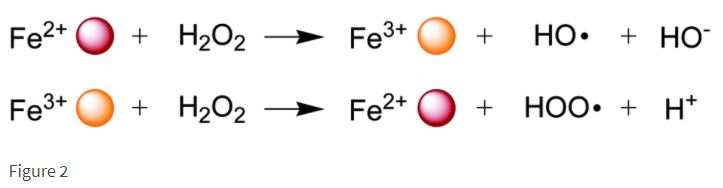
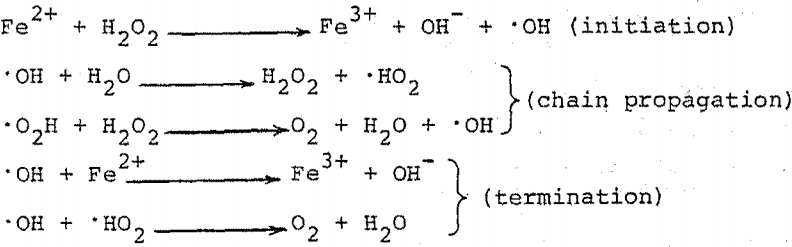Aqueous‐Phase Selective Oxidation of Methane with Oxygen over Iron Salts and Pd/C in the Presence of Hydrogen - Kang - 2019 - ChemCatChem - Wiley Online Library

Oxidation of Wine Phenolics: A Critical Evaluation and Hypotheses | American Journal of Enology and Viticulture

Decomposition of Hydrogen Peroxide and Organic Compounds in the Presence of Dissolved Iron and Ferrihydrite | Environmental Science & Technology

Objectives Define oxidation and reduction in terms of electron loss and gain. Deduce the oxidation number of an element in a compound. State the names. - ppt download
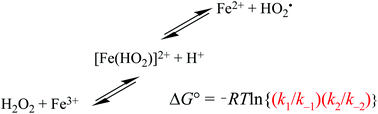
The principle of detailed balancing, the iron-catalyzed disproportionation of hydrogen peroxide, and the Fenton reaction - Dalton Transactions (RSC Publishing)

fenton reagent reaction hydrogen peroxide iron sulfate | Fundamental Photographs - The Art of Science

Activation of Hydrogen Peroxide by a Titanium Oxide-Supported Iron Catalyst: Evidence for Surface Fe(IV) and Its Selectivity | Environmental Science & Technology

Objectives Define oxidation and reduction in terms of electron loss and gain. Deduce the oxidation number of an element in a compound. State the names. - ppt download