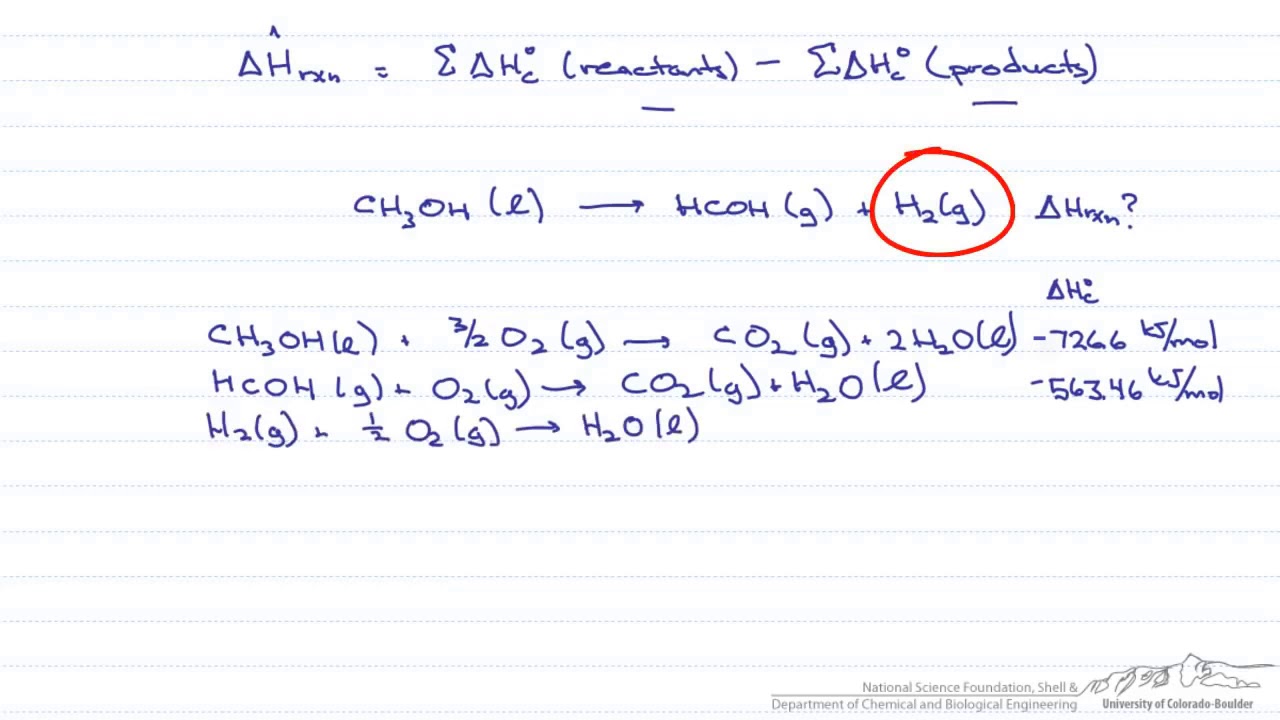![Total: 2 Average: 5/5] What is the heat of combustion? What is the definition of enthalpy of c… | Chemistry lessons, Chemistry classroom, Chemistry notes Total: 2 Average: 5/5] What is the heat of combustion? What is the definition of enthalpy of c… | Chemistry lessons, Chemistry classroom, Chemistry notes](https://i.pinimg.com/736x/99/83/b8/9983b8be657f09567f1e4b88e6bed700.jpg)
Total: 2 Average: 5/5] What is the heat of combustion? What is the definition of enthalpy of c… | Chemistry lessons, Chemistry classroom, Chemistry notes

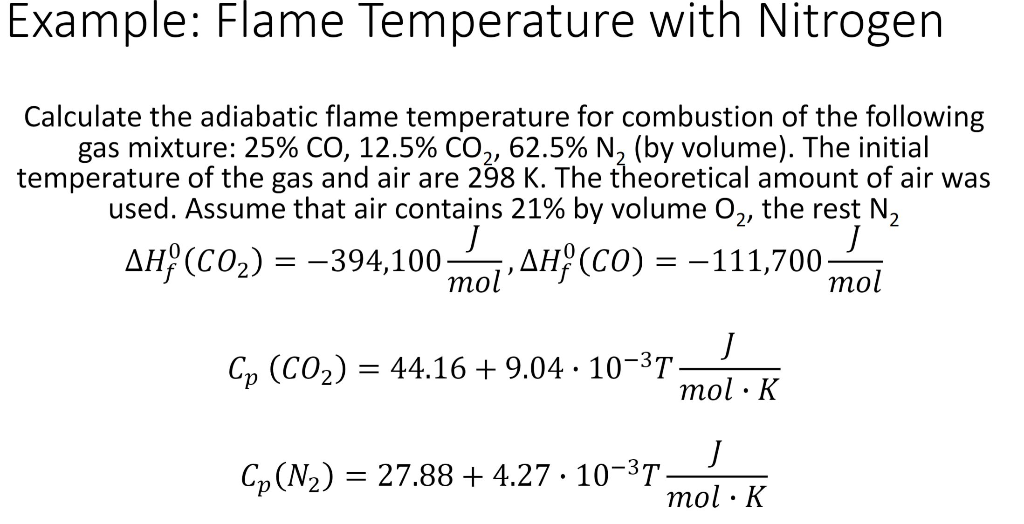
Adiabatic Flame Temperature Calculation for Ethane - Fire Protection Engineering (FPE) Teaching Tool - YouTube

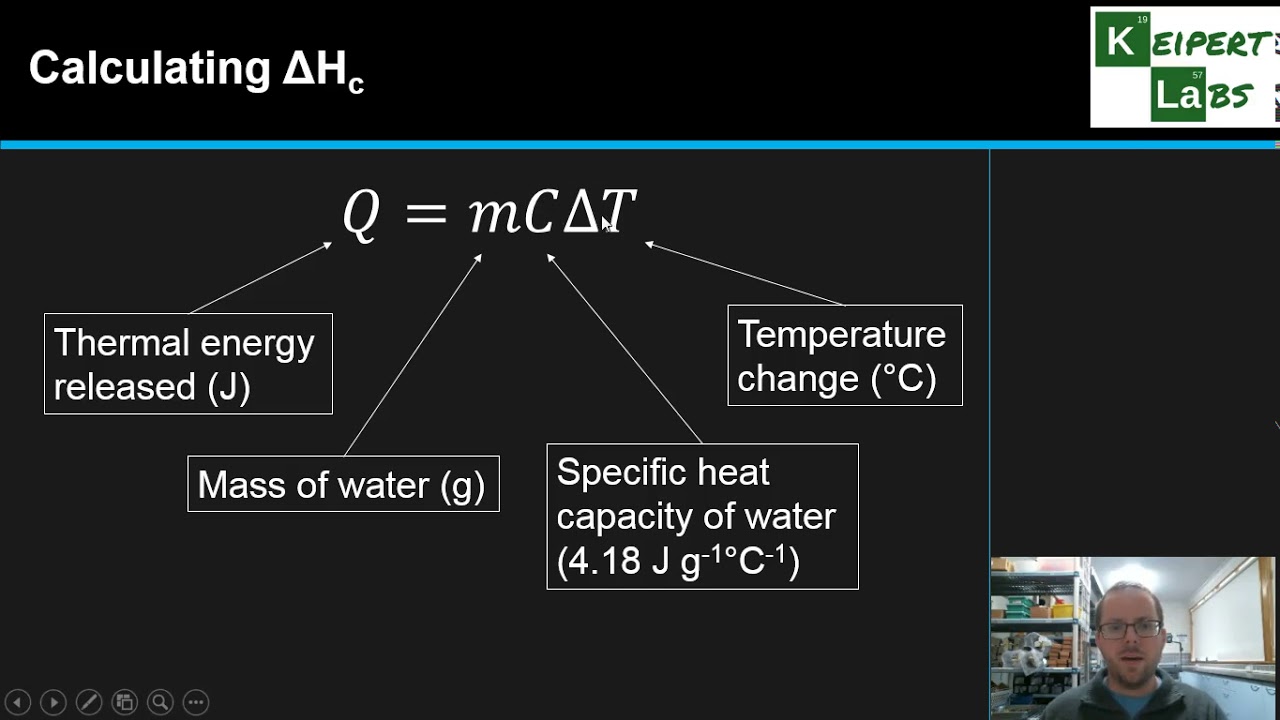
Molar Heat of Combustion Formula & Calculation | What is Heat of Combustion? - Video & Lesson Transcript | Study.com

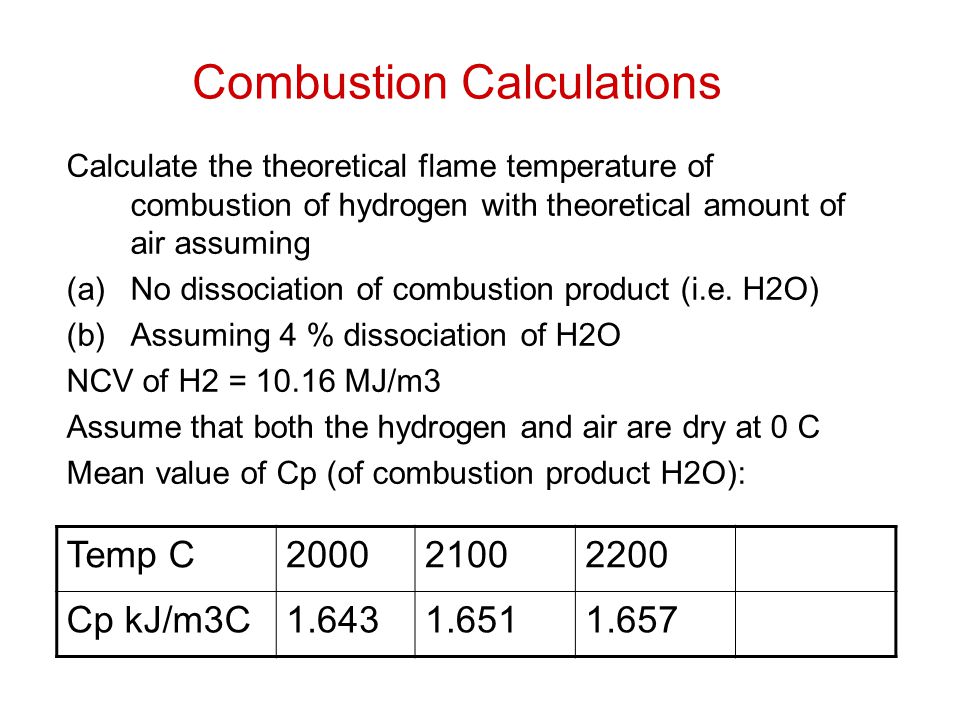
Combustion Calculations Calculate the theoretical flame temperature of combustion of hydrogen with theoretical amount of air assuming (a)No dissociation. - ppt download
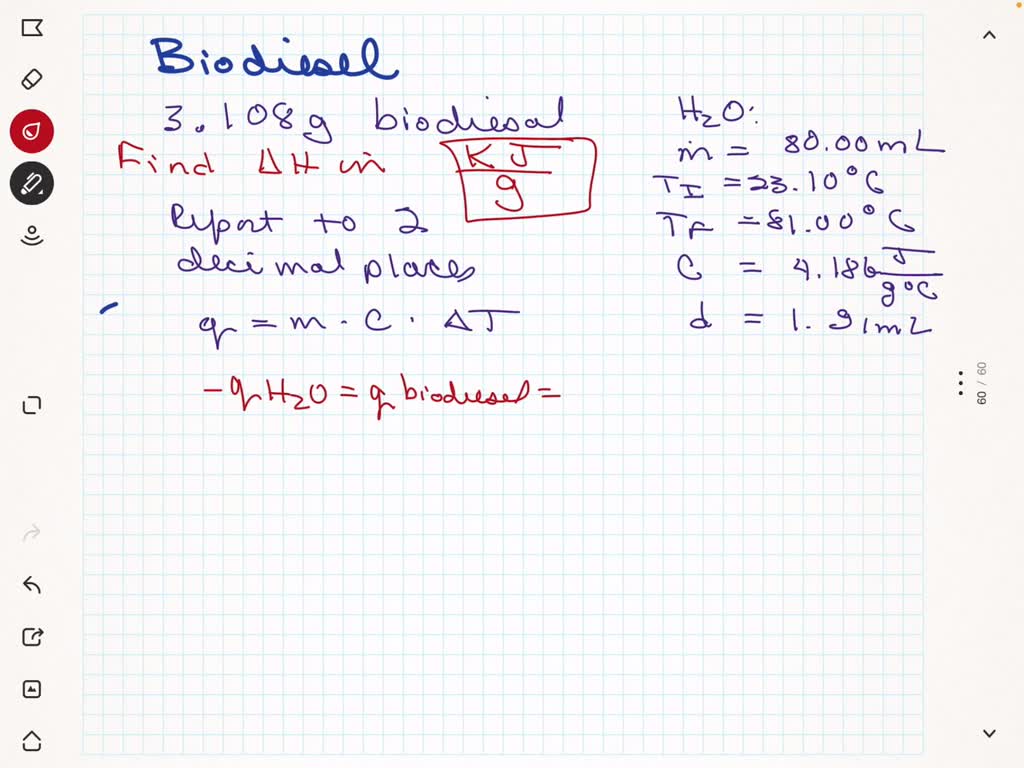
SOLVED: In the combustion of 3.108g of biodiesel made from lard, the temperature of 80.00 mL of water in a soda pop can increased from 23.10 to 81.00 degrees celsius. Calculate the