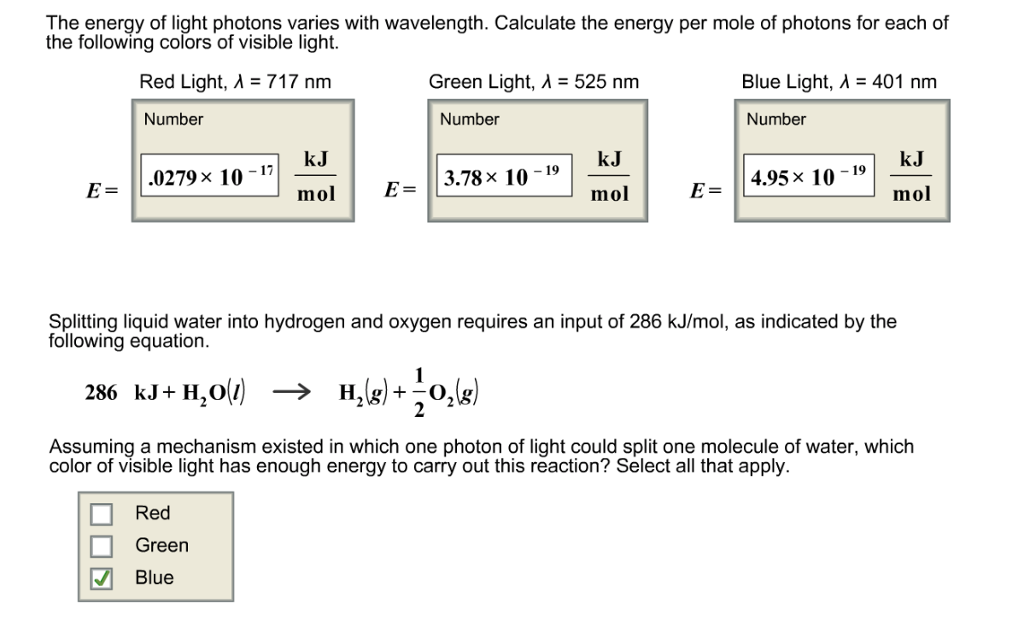
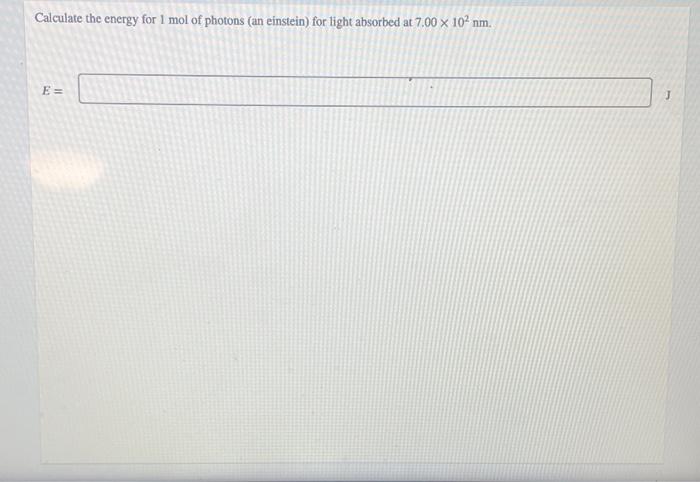
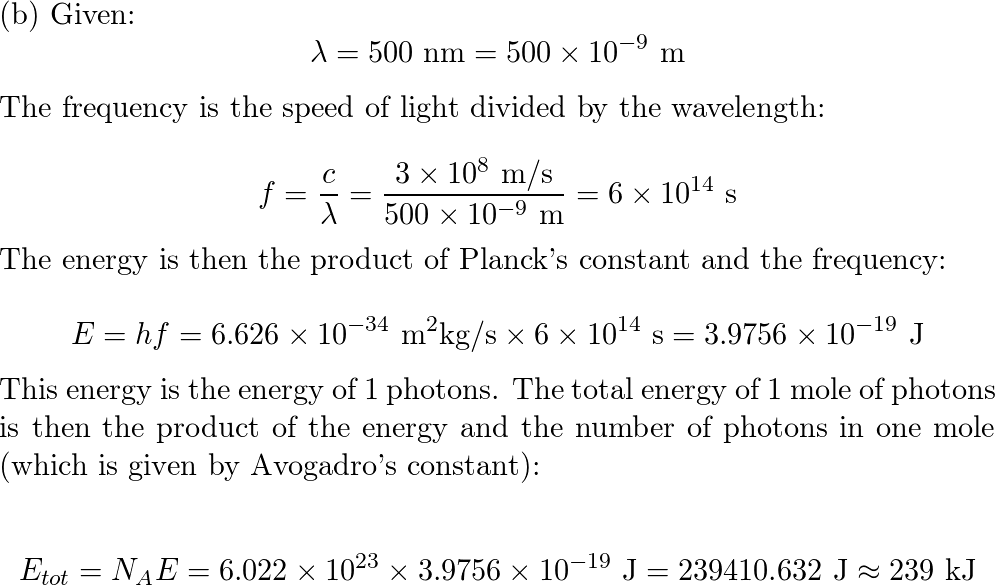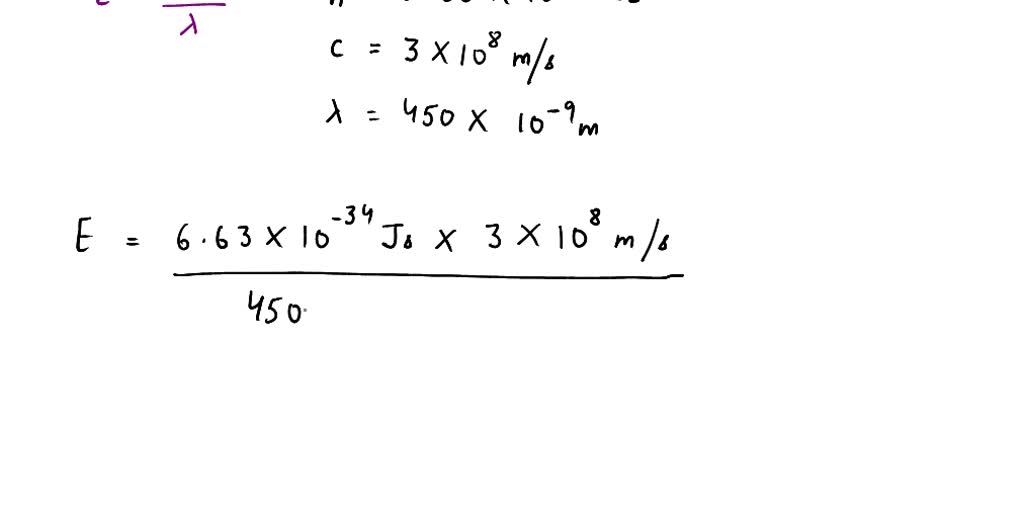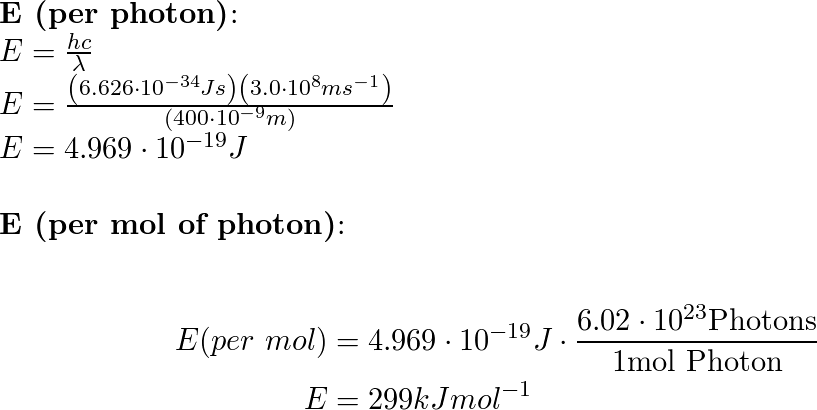SOLVED: determine the energy of 1.40 mol of photons for each. i have to convert to kJ...•infrared radiation 1540 nm•visible light 504nm•ultraviolet radiation 165 nm

OneClass: A photon has a frequency of 3.40 × 109 Hz. Calculate the energy (in joules) of 1 mole of p...

Calculate the energy of one mole of photons of radiation whose frequency is `5 xx 10^(14) Hz`. - YouTube
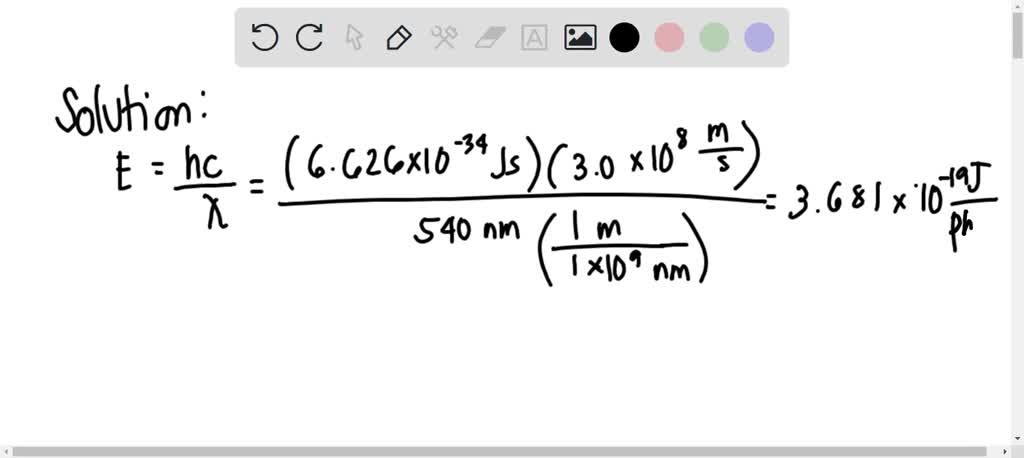
SOLVED: Calculate the energy in kJ/mole for a photon of light with wavelength 540. nm if n = 1. Group of answer choices 7.18x10-28 kJ/mole 6.46x10-11 kJ/mole 4.51 kJ/mole 222 kJ/mole 1.47
![Calculate the energy of 1 mol of photons an electromagnetic radiation of frequency 2.5xx10^(14)Hz." "[h=6.626xx10^(-34)J*s] Calculate the energy of 1 mol of photons an electromagnetic radiation of frequency 2.5xx10^(14)Hz." "[h=6.626xx10^(-34)J*s]](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/160817183_web.png)