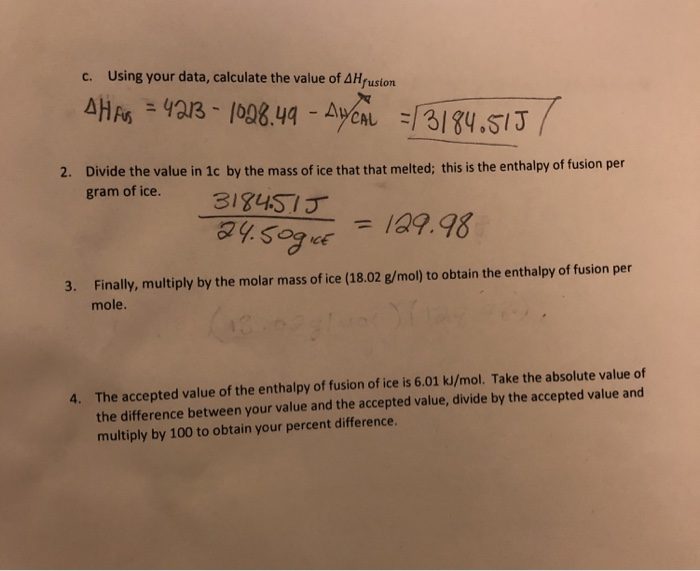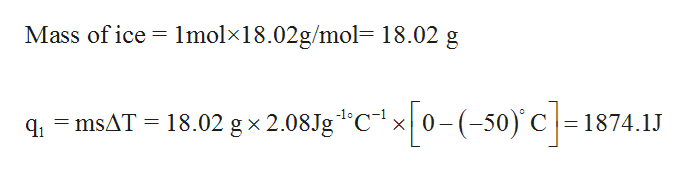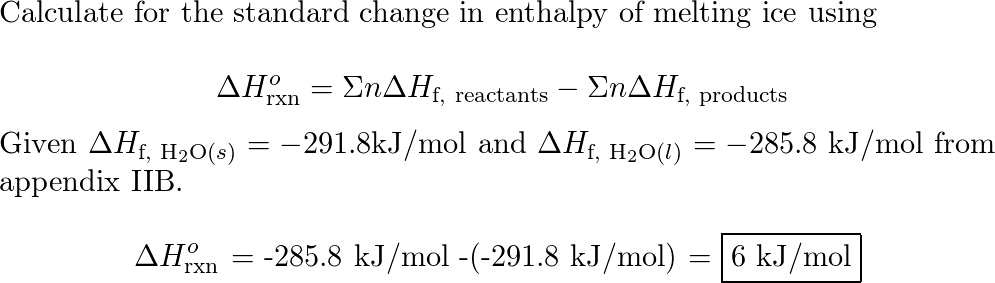SOLVED:Use standard enthalpies of formation to calculate the standard change in enthalpy for the melting of ice. (The ΔHf^∘ for H2 O(s) is -291.8 k k / / mol . ) Use

OneClass: Enthalpy of a Phase Change Heat, q, is energy transferred between a system and its surround...

The enthalpy of fusion of ice is 6.02kJ mol^-1 . The heat capacity of water is 4.18J(g^oC)^-1 . What is the smallest number of ice cubes at 0^oC , each containing one

Calculate the heat of fusion of ice from the following data of ice at `0^@C` added to water. Mass of - YouTube
How to calculate the mass of ice used in a change of state experiment when I had 50g of water, an 80J calorimeter, an initial temperature of 46C and a final temperature

SOLVED: Use standard enthalpies of formation to calculate the standard change in enthalpy for the melting of ice. (The H f for H2O(s) is -291.8 kJ>mol.) Use this value to calculate the