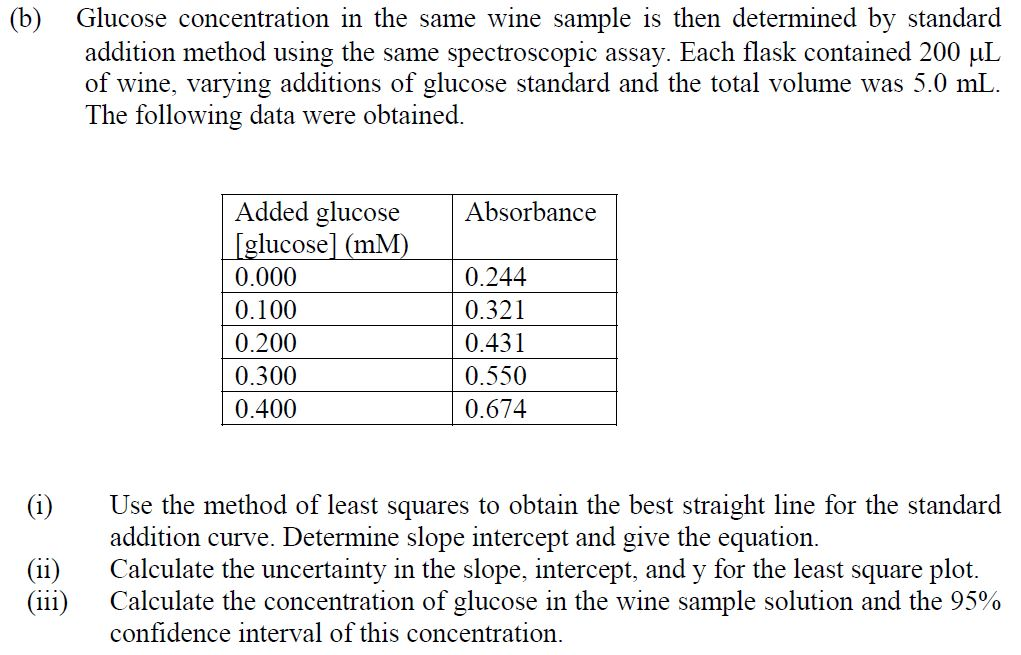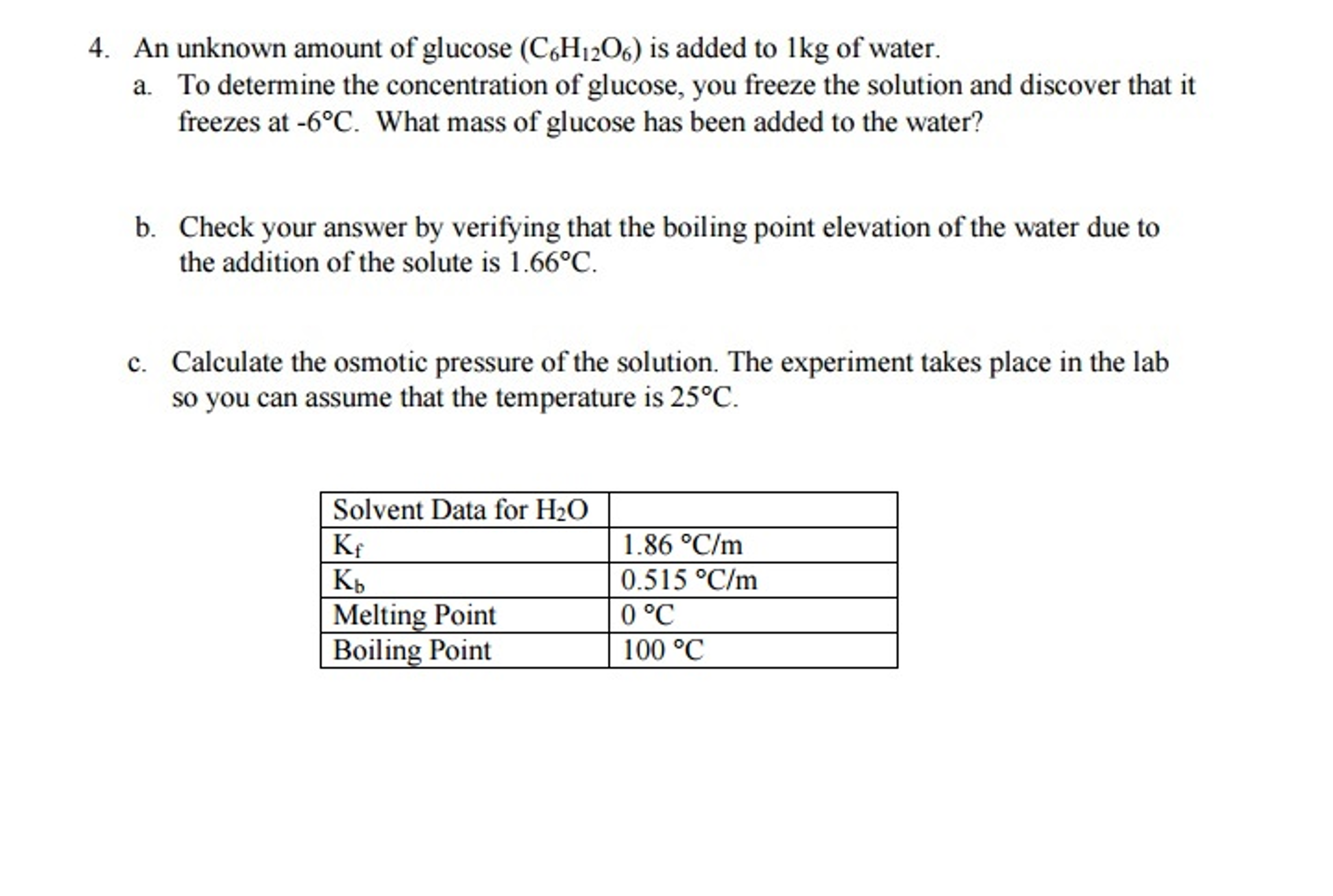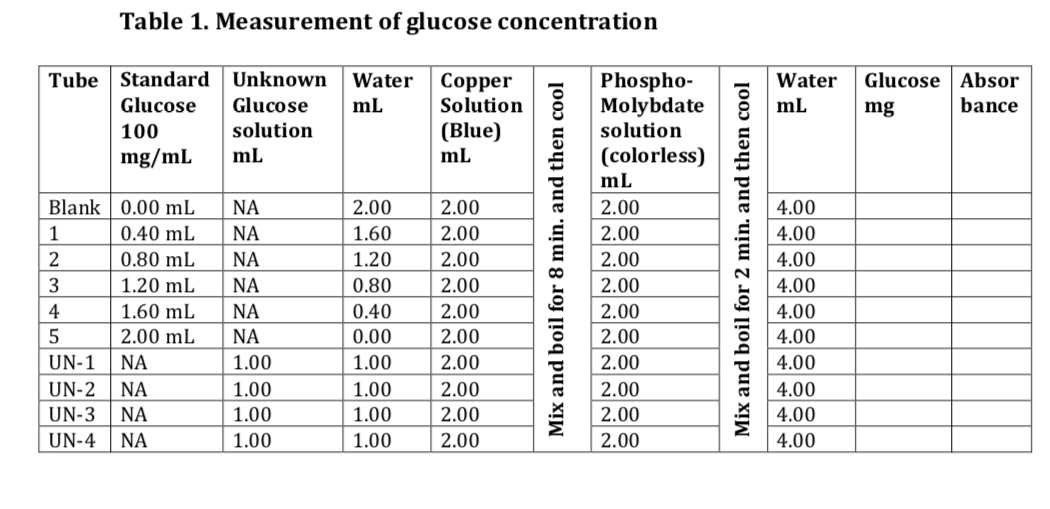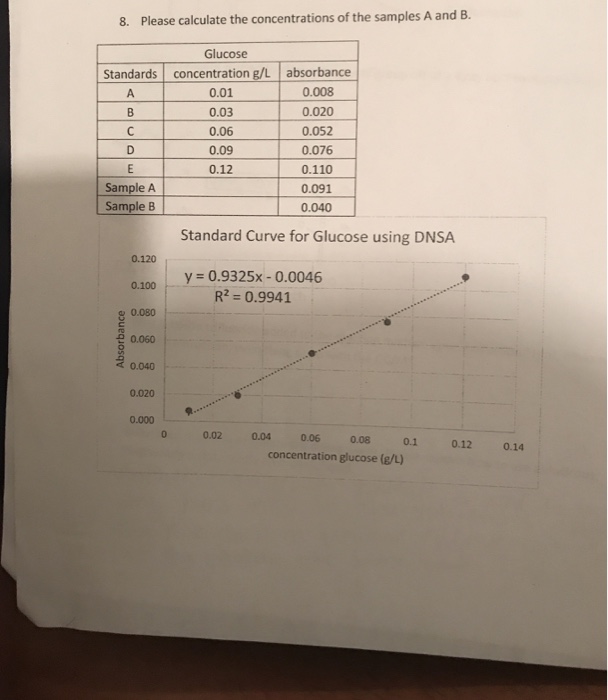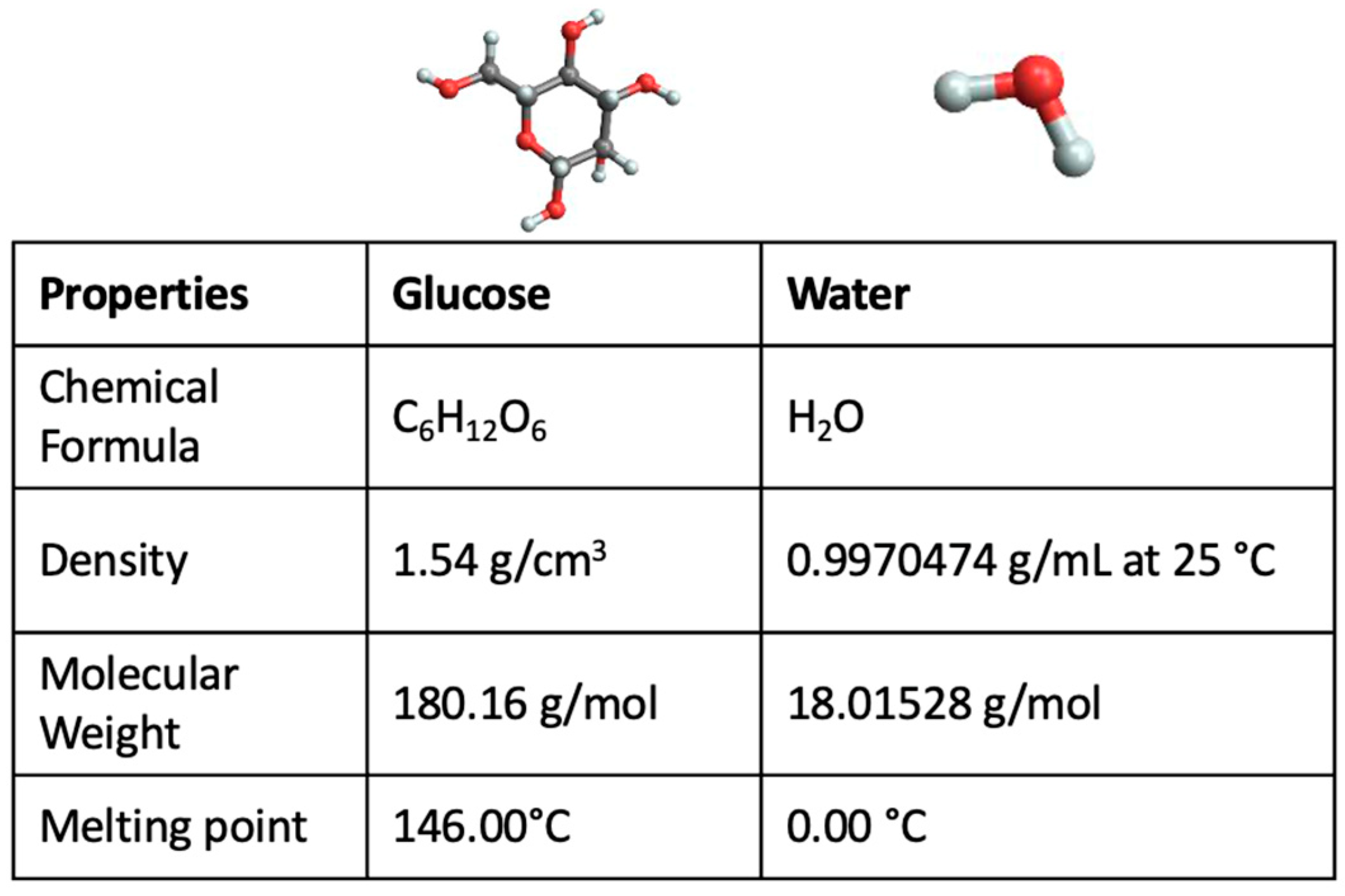
IJMS | Free Full-Text | Investigation of Glucose–Water Mixtures as a Function of Concentration and Temperature by Infrared Spectroscopy

A solution is made by dissolving 50 g of glucose in 250 g of water calculate the concentration of the solution in mass percentage

The molarity of a glucose solution containing 36g of glucose per 400mL of the solution is: - YouTube

SOLVED: 18-20.A 34.2 g of glucose is dissolved in 400 grams of water. Calculate the percentage by mass concentration of glucose solutions

SOLVED: 65 grams of glucose dissolved in 435 grams of water. Calculate the concentration of the solution in terms of mass by mass percentage.

SOLVED: Calculate % concentration of & solution prepared by dissolving 8.5 26.5 9 of - 9 of glucose water. Round result to (C6H120s) three significant digits. 32.1 24.3 3.21 2.43



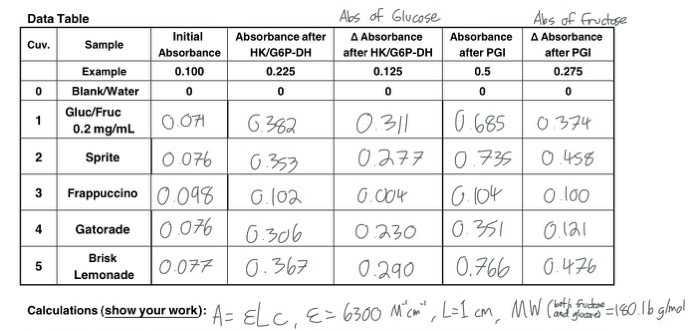

.jpg)